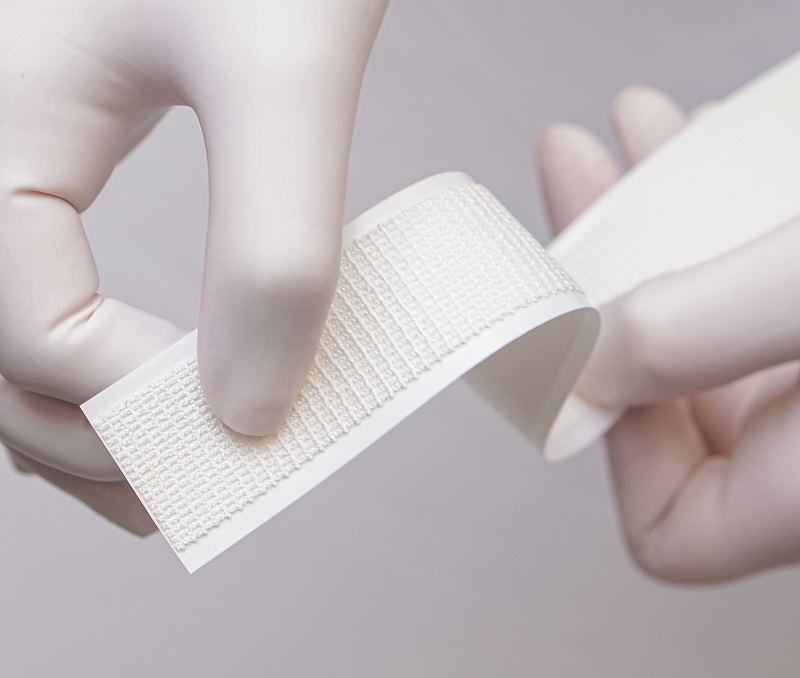
Sylke Inc has launched SYLKETM, the first dressing made of pure hypoallergenic silk fibroin.† SYLKE™ is designed to enhance healing across surgical wounds and to reduce complications and infections which can lead to poor scarring outcomes.1,2 Based on head-to-head data* published today in the Aesthetic Surgery Journal (ASJ) Open Forum

SYLKE ™ adhesive wound closure prototype outperformed DERMABONDTM PRINEOTM (p<0.001) across all measures, significantly reducing the incidence of medical adhesive-related skin injuries (MARSIs). The study was published in collaboration with The Johns Hopkins University School of Medicine and the University of California San Diego.
Each year, 1.5 million Americans suffer from MARSIs caused by synthetic surgical adhesive dressings, leading to painful complications, including surgical site infections (SSIs) and poor scarring outcomes. The related cost to the US healthcare system is $3.3 billion each year. The current standard of care, including inflexible tapes, synthetic materials, and aggressive liquid glue medical adhesives often lead to skin tears, blisters, cell damage and allergic contact dermatitis (ACD).These problems are often overlooked, vastly under-reported and preventable.“SYLKE ™ aims to revolutionize surgical wound care by eliminating medical adhesive-related skin injuries that lead to complications such as infections and poor scarring outcomes.

We see a benefit to millions of patients and aim to decrease the financial burden on the healthcare system by bringing SYLKETM to market,” said Dr. Mark Mofid, MD, FACS, an assistant Professor of Plastic Surgery at The Johns Hopkins University School of Medicine and inventor of SYLKETM.
“Scarring from surgical procedures dramatically affects aesthetics and impacts patients’ mental health and overall well-being. We are especially pleased to extend the art of scar-minimizing wound closure to all healthcare professionals, patients, consumers and military personnel.”
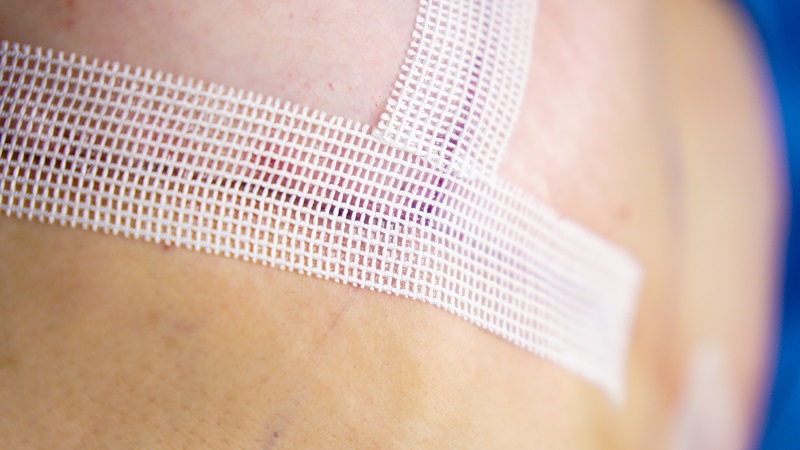
SYLKE™ is commercially available and can be purchased over-the-counter by healthcare professionals and the general public through www.sylke.com. Five-unit introduction boxes will be launched at the cost of $115/strip 3⁄4 up to 32 percent less than a current standard of care 3⁄4 with volume discounts for large-scale buyers, such as hospital systems and clinics.
About SYLKE ™
SYLKETM Adhesive Wound Closure is the first premium, naturally sourced, and water-resistant “all-in-one” system designed with hypoallergenic materials to enhance wound healing and reduce surgical site complications to improve patient outcomes.
Made of pure silk fibroin, the SYLKE™ patented breathable mesh design gently adheres seamlessly to irregular wound surfaces remaining water-resistant for up to two weeks withoutthe need for reapplication. The inherent elasticity of the mesh design allows for SYLKE™ to minimize shear forces on the skin as a result of swelling or motion while maintaining precise wound edge approximation during the healing process. SYLKETM is available as a 2.5 cm wide strip with an easily peeled paper backing that can be effortlessly cut and applied within seconds to post-surgical incisions, cuts, or simple lacerations thus eliminating the need for additional applicators, glues, dressings or devices.
SYLKETM was registered as an FDA Class 1 medical device in October 2023.
For additional information, please visit www.sylke.com.

























